Mudskippers: An Introduction For Aquarists
By Gianluca Polgar
Mudskippers are members of the subfamily Oxudercinae, a group within the family Gobiidae. The Oxudercinae presently includes 40 species across 10 genera, but only 32 species within 5 genera are usually referred to as “mudskippers”. The five genera are Boleophthalmus, Periophthalmodon, Periophthalmus, Scartelaos and Pseudapocryptes.
Mudskippers have stirred popular and scientific imagination since the 17th century because of their extraordinary adaptations to an amphibious lifestyle. Scientists have been particularly impressed by their large and mobile eyes, and many mudskipper scientific names reference these wonderful structures. For example, the name Oxudercinae comes from the genus Oxuderces, coined by Eydoux & Souleyet in 1850, which comes from the Greek word okyderkes, meaning “sharp-sighted”. The genus Periophthalmus gets its name from the frog-like position of its eyes that allows it superb all-around vision, peri meaning “around” and ophtalmos meaning “eye”.
Distribution
Mudskippers are mostly tropical to subtropical animals, and are distributed across a region from the Atlantic coast of Africa as far east as the Pacific islands of Samoa and Tonga. The most widely distributed and species-rich genus is Periophthalmus, within which are currently accounted 18 species. The only mudskipper from the Atlantic is a member of this genus, Periophthalmus barbarus, and some Periophthalmus species have ranges that extend into the temperate zones of southern Japan and eastern Australia, where they overwinter inside deep burrows.
A few other mudskippers are found in these temperate zone environments as well, including species of Boleophthalmus and Scartelaos, but otherwise mudskippers are typically animals of hot, humid mangrove forests and tidal mudflats. They are mainly found within the intertidal zone, and besides being able to move about on land, all mudskippers share an ability to adapt to rapid changes in salinity. Some species of the genera Periophthalmus and Periophthalmodon are also found in the supratidal zone, which is covered by sea water only during extreme tides. Here they can live in back-mangrove habitats or freshwater swamps, being adapted to almost full fresh water.
'Figure 1' - A frequently traded mudskipper, Periophthalmodon septemradiatus, in a paludarium. © Gianluca Polgar
Adaptations to life on land
Because they spend so much time on land, mudskippers need to be able to breath in air. Like frogs and salamanders, they have a rich network of capillaries just under their skin that allows oxygen to diffuse into the blood and carbon dioxide out. This type of breathing is known as cutaneous respiration. Special mucous protects the skin and minimises water losses.
Together with cutaneous respiration through the skin of the body, when mudskippers are out of the water they also mostly breathe through their mouths and throats, which are wide, moist, and lined with capillaries, rather like a lung. When they gulp air, mudskippers seal the gill chamber with a special valve to minimise water losses and expand their gill covers so that the mouth and throat are as large as possible.
Surprisingly perhaps, the adaptations mudskippers have for air breathing are not as complex as those seen in some other fish, such as lungfish or gouramis. Apparently air breathing hasn't played a major role in the evolution of mudskippers into amphibious fish able to operate successfully on land. Several studies suggest that other physiological and anatomical adaptations, including osmoregulation, excretion and locomotion on land, have been rather more important.
'Figure 2' - External anatomy of a mudskipper, Periophthalmus variabilis. © Gianluca Polgar
Adaptations to semi-terrestrial conditions are so extreme that they have lost some of the abilities typical of fishes. For example, some mudskipper species are incapable of repaying an oxygen debt while under water. In other words, they are effectively short of breath when under water, and have to slow down their heartbeat and metabolic activity, rather like diving air-breathing animals including seals, dolphins and (without SCUBA gear) humans.
Mudskippers see well out of water, but are short-sighted under the waterline. When they are on land, their eyes can be retracted and moistened in the fluid-filled dermal cups below the orbits. This makes them the only fishes on Earth capable of blinking! Mudskippers seem also to be able to hear sounds propagated through the air, and will react to things like the buzzing of flies. Other fish detect sounds underwater using ears within their skulls (comparable to our inner ear) and the lateral line system running along their flanks, but which organ mudskippers use to detect sounds moving through air is unknown.
Behavioural adaptations
Many of their most striking adaptations to life on land are behavioural. Since they are very mobile and constantly move between land and water, mudskippers need to cope with extreme changes in temperature, humidity and salinity. But at the same time mudskippers are different from other fish in intertidal habitats in being able to move from a stressful area to one that is more favourable. For example, other tide-pool fish cope with an increase of water temperature by adjusting their metabolism, but a mudskipper will leave the water and allow its body to cool down through evaporation. Should it lose too much moisture this way, the mudskipper will dive back into the water to get wet again. If there isn't a pool nearby, the mudskipper will instead roll in wet mud.
Mudskippers have a variety of locomotory behaviours that enable them to move in water and on land. Beside straightforward swimming, they will paddle just below the waterline with only their eyes emerged, or even skim across the surface. On land their modes of locomotion range from the relatively sedate crutching through to energetic jumping. Mudskippers are good at climbing, too.
Like many gobies, mudskippers are proficient burrowers. They dig deep burrows into soft substrates, and will seek refuge in their burrows from predators and extremes environmental conditions including, as mentioned earlier, periods of cold. Their burrows are also important for breeding, since eggs are laid inside the burrow and, when this could be observed, protected by the male. Given that mudskippers aren’t very good at breathing underwater, for many years it was unknown how they could remain in their water-filled burrows for long periods while brooding their eggs or taking shelter during high tide. In fact, the water inside the burrow has extremely low oxygen levels. It was eventually discovered that mudskippers gulp air and then carry it down into their burrows, where the air is released into special chambers. This behaviour is likely especially important for the proper development of the eggs, since these are usually laid on the ceiling of the same chamber. So besides protecting the eggs, the male mudskipper also ensures the developing eggs are kept in a moist, oxygen-rich environment. Recently, some Japanese researchers found that male Periophthalmus modestus flood this chamber when the eggs are ready to hatch, allowing the larvae to leave the nest during high tide.
Advanced bony fish, including most gobies, use suction to obtain their food. By expanding the mouth rapidly, water is drawn into the buccal cavity and throat, and whatever prey or food particles are in front of the fish will be sucked in along with water. This won’t work on land though, so mudskippers feeding on land have to work in a different way. The largely carnivorous species such as Periophthalmus spp. have sharp teeth to grab their prey, while modified pharyngeal jaws grip the prey and pull it down into the oesophagus.
Herbivores such as Boleophthalmus spp. feed in a different way. They scrape the watery film of algae and mud on the sediment, using funny side-to-side movements of the head. When they have collected enough of this material, they find a pool of water, and use this to sieve the mixture in their wide pharyngeal jaws, as if they were panning for gold.
Reproduction
In the few investigated mudskipper species, we do know that mating invariably takes place in the male’s burrow. In some species the female is driven out after mating and the male assumes broodcare responsibilities, but in others a cohabitation period of variable length occurs. In this regard they are much like the majority of gobies, with broodcare by the male being typical. But much of mudskippers’ life cycles remains a mystery. Not much is known about the biology of mudskipper larvae; in some species the larvae abandon the nest after hatching, and drift about in the marine plankton before they metamorphose and settle down in intertidal areas as juvenile mudskippers. It’s unknown how long larval mudskippers drift in the plankton for, or how far this allows larvae mudskippers to travel.
External anatomy and identification
The basic anatomical features for identification are illustrated in Figure 2. Most adult or subadult mudskippers can be easily discriminated to species level by examining a combination of characters (colouration patterns of the body and fins, and shape of the pelvic fins: Figures 2 and 3). However, these traits are not easily observed in a fish sitting on a stone in your tank. There are some easy techniques to have a closer look or even gently handle your mudskipper to know more
![]()
'Figure 3' - Pelvic fins of some species of the genus Periophthalmus (ventral vi ew, fishes are facing upward). Mudskipper species exhibit various degrees of fusion of their pelvic fins: A: Periophthalmus chrysospilos; B: Periophthalmus darwini; C: Periophthalmus modestus; D: Periophthalmus variabilis; E: Periophthalmus argentilineatus (drawings not to scale). A-D: a pelvic frenum (a fold of skin similar to the tiny one between your fingers) unites the pelvic spines, while a basal membrane of variable extension (decreasing from A to D) connects the innermost rays of the pelvic fins; E: absence of both the pelvic frenum and basal membrane. © Gianluca Polgar
Having an idea of where your fish comes from will be an obvious advantage, so ask your retailer. Presented here is a synopsis of the most commonly traded species, but it must be remembered that body colouration patterns are variable in a live individual, e.g. the dark saddle-like banded pattern on dorsum and flanks, depending on various behavioural and physiological factors. Therefore you should never rely on a single trait like colouration, but instead cross-check as many different characteristics as you can.
Most commonly traded species
Most of the mudskippers traded commercially are species of Periophthalmus and to a lesser degree Periophthalmodon. These are the species described in the following sections. Only occasionally traded are species such as Pseudapocryptes elongatus, Boleophthalmus boddarti and Oxuderces dentatus. These latter species are finicky specialists that feed mostly or exclusively on microscopic algae skimmed from mud, and might not be easy to rear in most aquarium conditions, largely explaining why they are so rarely seen in aquarium shops.
Mudskippers are regularly exported from West Africa, Singapore, Vietnam, Thailand, and in smaller numbers, India and Sri Lanka. This is why for example Australian or New Guinean species are almost impossible to find in an aquarium shop. Nonetheless, the author’s experience is limited to the European, North American and Southeast Asian markets: other species might be available in different countries hosting fervent aquarist communities, such as Japan and Australia.
Mudskippers are frequently misidentified by retailers, or given obsolete names (synonyms) different to those used in the modern literature. Consequently any names supplied by the retailer should be accepted only provisionally, and when possible compared against the scientific literature. Even though some work remains to be done, the present taxonomy of this group is relatively stable and generally accepted.
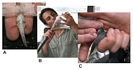
'Figure 4' - How to handle a mudskipper to identify it. A: try to delicately confine the mudskipper with your hand against the glass of the aquarium, below water (better if the mouth is out of water, to let him breathe more efficiently): this will allow you to examine its pelvic fins at a close distance. B: Alternatively, observe him from below when he climbs on the walls of a transparent plastic bag. C: the best way to get more details is probably a close examination in your hand, even if this procedure should always be avoided when there are other alternatives, in order to avoid unnecessary stress for the animal; hold the mudskipper out of water in your palm with three fingers, covering its head and eyes. In the darkness, he will eventually stop fighting, allowing a closer examination. Be particularly careful when unfolding the delicate fin membranes to observe their colouration. For smaller specimens, a small wet brush may be utilised. © Gianluca Polgar
Sex determination
Sex discrimination of adult mudskippers is not straightforward. Clear-cut sexual dimorphism is exhibited by only few species (e.g. Periophthalmodon septemradiatus, Figure 6; Periophthalmus novemradiatus, Figure 10). If this is not the case, the only external clue is the shape of the urogenital papilla, as in most gobies: pointed in males and square and tapered in females (Figure 5).
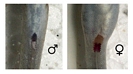
'Figure 5' - A clear example of a male (left) and female (right) urogenital papillae (Periophthalmus gracilis, from Peninsular Malaysia; ventral views; specimens are facing upward; the female is full of eggs). The profile of the papilla was contoured by dashed lines. Unfortunately, things are not always that easy, especially in subadults, but also in sexually inactive adults. © Gianluca Polgar
Diet
All the species of the genera Periophthalmus and Periophthalmodon are opportunistic carnivores, though some species also (accidentally?) ingest some algae as well. In general they are gape-limited, meaning they will eat whatever they can catch and swallow! In some species, and within some populations, seasonal specialisation may exist, presumably a response to changes in prey availability.
Under aquarium conditions these mudskippers have proven to be very adaptable, though small live foods such as flies, spiders and so on are particularly favoured. Once settled, wet-frozen foods and even dry pellets are readily accepted, and captive mudskippers may become tame enough to be fed by hand.
Species descriptions
Periophthalmodon septemradiatus (Hamilton, 1822)
'Figure 6a' and 'Figure 6b'


Periophthalmodon septemradiatus (A: female; B: male), Kuala Selangor, Selangor, Malaysia; © Gianluca Polgar. In this and other photos, scale bars are 10 mm long.
'Figure 7'
Synonyms: Gobius tredecemradiatus, Gobius septemradiatus, Periophthalmodon tredecemradiatus, Periophthalmus borneensis
Maximum recorded size: 100 mm standard length (SL, i.e., from the tip of the snout to the structural base of the caudal fin).
Sexual dimorphism: Females have extremely reduced first dorsal fins.
Body colouration: Body background colour grey to brownish, dorsally paler, white on venter and throat; margins of opercula dark; a brown stripe is frequently visible, running dorsally and posteriorly from each orbit to the end of the first or second dorsal fin, becoming a row of irregular dark blotches, up to the caudal peduncle; these two series of dark dorsolateral blotches may form a pattern of 8-10 saddle-like bars; numerous small dark brown, pale red and pale blue speckles are present on snout, opercula and flanks; scales on opercula are arranged in a typical diagonal pattern and have dark margins.
Fin colouration: First dorsal fin blackish to dark blue with reddish margin in males; second dorsal fin dusky with series of dark speckles on rays and a red margin; pectoral fins greyish with red speckles on rays; caudal fin dusky with series of dark speckles on rays.
Pelvic fin shape: Pelvic fins are completely separated and no pelvic frenum is present (Fig. 3, condition E)
Distribution: This species is distributed in East India, Myanmar and SE Asia; it is frequently imported from Viet Nam. It is probably one of the most terrestrial species, living in almost pure freshwater, even nearby the small irrigation dikes crossing human villages. It is one of the few mudskippers which flees to the land when it is chased, plunging into water only as a last resource. This species is extremely cryptic: if not moving, it is almost invisible on the forest ground. In Malaysia Pn. septemradiatus is probably rare, maybe due to the destruction of mangrove forest and peat swamps from land; up to date, it has been found only in a village nearby Kuala Selangor. It probably feeds on small invertebrates. Its behaviour is completely unknown.
Periophthalmus argentilineatus Valenciennes, 1837; Silver-lined Mudskipper, Barred Mudskipper
'Figure 7' - Periophthalmus argentilineatus, Morib, Selangor, Malaysia © Gianluca Polgar.
Synonyms: Euchoristopus kalolo regius, Periophthalmus dipus, Periophthalmus sobrinus, Periophthalmus vulgaris
Maximum recorded size: 93 mm SL.
Sexual dimorphism: None known.
Body colouration: Body background colour brownish to dark grey on dorsum and sides, ventrally whitish; head ventrally white; many small white speckles on cheeks, opercula and sides of body; silvery vertical stripes on flanks, ventrally more evident; 3-8 dorsal dark brown saddle-like irregular bars, may also be visible.
Fin colouration: First dorsal fin with a reddish brown background, a wide black to dark brown stripe below margin, darker in the anterior portion, and many small white spots below it; margin white to transparent; second dorsal fin with a transparent background, a black medial stripe, irregular brownish speckles proximally on rays and membrane, and an orange margin; caudal fin dusky, with series of dusky to brownish speckles along rays.
Pelvic fin shape: Pelvic fins are completely separated and no pelvic frenum is present (Fig. 3, E).
Distribution: The Silver-lined Mudskipper is the most widespread mudskipper species, presently known from East Africa eastward to the entire Indo-Pacific region, to the Samoa and Tonga Islands, southern Japan and eastern Australia. This species is particularly popular in southern Japan. It is usually found nearby water at low tide, frequently nearby the mangrove marine fringe, but it apparently is an extremely adaptable species, and different populations are found in different environmental conditions. A very territorial species: aggressive and agonistic behaviours are frequently observed. Males jump to attract females into burrows. Burrows typically have two main turreted openings. It feeds on small invertebrates. Its behaviour was studied in East African and Malagasy populations.
Periophthalmus barbarus Linnaeus, 1796; Atlantic Mudskipper, West African Mudskipper

'Figure 8' - Periophthalmus barbarus, Kribi, Cameroon, Africa; photo courtesy of R.E. Brummett.
Synonyms: Gobius barbarus, Gobius koelreuteri, Periophthalmus papilio, Periophthalmus erythronemus, Periophthalmus gabonicus
Maximum recorded size: 250 mm SL.
Sexual dimorphism: None known.
Body colouration: Background colour dark brown to greyish on head, dorsum and flanks, ventrally whitish; sky blue speckles on flanks, which are larger and more numerous on snout, cheeks and opercula; several black to dark brown irregular saddle-like diagonal bars may be visible on dorsum.
Fin colouration: First dorsal fin membrane dark brown, with a black to bluish band below margin, sandwiched by a narrow and broken whitish-blue stripe and a narrow transparent margin; completely black in some specimens. Second dorsal fin background brown, with a medial black stripe sandwiched by two white stripes, and a brown margin. Caudal fin dusky, with series of dark brown speckles along rays in some specimens; anal fin from whitish to brownish with a yellowish margin; pelvic fins dark brown on dorsal side; pectoral fins dusky.
Pelvic fin shape: Pelvic fins are completely separated and no pelvic frenum is present (Fig. 3, condition E)
Distribution: The Atlantic Mudskipper is the only mudskipper found on the Atlantic coast of Africa, its range extending from Morocco to Angola. If a mudskipper comes from this region, either it is a new species, or it is Periophthalmus barbarus. In Nigeria, its diet apparently includes algae in the dry season. In this region, it is found in mangrove and Nypa palm (Nypa fruticans) forests, and has consequently been included on lists of endangered species due to the high level of coastal environmental degradation in this area.
Periophthalmus gracilis Eggert, 1935; Slender Mudskipper
'Figure 9a' and Figure 9b'


Periophthalmus gracilis (A: female, B: male), Kukup Island, Johor, Malaysia © Gianluca Polgar
Synonyms: None.
Maximum recorded size: 45 mm SL.
Sexual dimorphism: Mature males and females have differently coloured dorsal fins; see below.
Body colouration: Body ground colour dorsally and laterally grey, ventrally white; white irregular blotches on ventral side of head and vertical iridescent bluish stripes on flanks; 5-7 diagonal, irregular saddle-like dark brown bars on dorsum.
Fin colouration: First dorsal fin with transparent background, with a faint greyish infra-marginal stripe, medially yellowish in mature males, and a large black blotch posteriorly, covering the last 3-4 spines; in some specimens, black spots may be present between spines; second dorsal fin with a transparent background, grey spots on proximal membrane and a medial dark stripe; a reddish margin is present in mature males; membrane of caudal fin and pectoral fins transparent, with series of dark speckles on rays.
Pelvic fin shape: Pelvic fins are completely separated and no pelvic frenum is present (Fig. 3, condition E).
Distribution: The Slender Mudskipper is distributed from the Straits of Malacca to the Philippines and northern Australia. It is frequently imported from Singapore and Thailand. It is one of the smallest mudskipper species. It lives inside mangrove forests; at higher levels, reached by tides only intermittently; it is frequently found around tide pools or the openings of burrows made by the Giant Mudskipper, Periophthalmodon schlosseri. It feeds on small invertebrates, but otherwise its behaviour is almost completely unknown.
Periophthalmus novemradiatus (Hamilton, 1822); Indian mudskipper
'Figure 10' - Periophthalmus novemradiatus, shot in the field ( Thailand), unknown provenance © Yuko Ikebe
Synonyms: None.
Maximum recorded size: 51 mm SL.
Sexual dimorphism: The first spine of the first dorsal fin is highly elongated on males, reaching the second dorsal fin if pushed down.
Body colouration: Body ground colour dorsally and laterally grey to pale brown, whitish on venter; white speckles scattered on flanks, denser and rounded on opercula and cheeks, interspersed with smaller dark dots only on flanks; speckles on cheeks can become sky blue; 5-8 diagonal, irregular saddle-like dark brown bars may be visible on dorsum.
Fin colouration: First dorsal fin membrane with a white margin, red between the first 5-7 spines, with brown diffused blotches in the distal portion, hyaline to grey for the rest, and with white flecks parallel to the spines in some specimens; spines not pigmented; second dorsal fin with a dusky translucent background, white margin, a large dark grey inframarginal stripe, a medial dark stripe and coalescent dark grey spots on the proximal membrane; membrane of caudal fin and pectoral fins dusky, darker along rays. Pelvic fins dorsally pigmented and with a white margin.
Pelvic fin shape: Pelvic fins are fused by a basal membrane for more than half the length of the inner pelvic rays, and a strong pelvic frenum is present (Fig. 3, condition C-D)
Distribution: The Indian Mudskipper is distributed in the Gulf of Bengal, from East India to southern Thailand, where it may occur with Periophthalmus variabilis. This species was found not far from the water’s edge, along the mud banks of creeks and tidally influenced rivers. Its behaviour and ecology are largely unknown.
Periophthalmus variabilis Eggert, 1935; Dusky-gilled Mudskipper
'Figure 11' - Periophthalmus variabilis, Carey Island, Selangor, Malaysia © Gianluca Polgar
Synonyms: None.
Maximum recorded size: 65 mm SL.
Sexual dimorphism: None known.
Body colouration: Body ground colour dorsally and laterally brown, ventrally whitish to grey; edge of the gill cover posteriorly blackened; head and trunk with numerous dark brown blotches, larger on trunk; tiny iridescent bluish speckles on cheeks and flanks; 5-8 diagonal, irregular saddle-like dark brown bars on dorsum.
Fin colouration: First dorsal fin with a transparent background with proximal red spots between elements, a greyish stripe below margin, anteriorly darker, and a translucent margin; second dorsal fin with a proximal series of reddish-brown spots, a dark stripe below margin, and a yellow margin; caudal fin membrane dusky, rays distally orange with series of brownish speckles; pectoral fins distally orange.
Pelvic fin shape: Pelvic fins are fused by a basal membrane for less than half the length of the inner pelvic rays, and a strong pelvic frenum is present (Fig. 3, D).
Distribution: The Dusky-gilled Mudskipper is distributed across Southeast Asia, and is frequently imported from Singapore, together with Periophthalmus gracilis. It is a relatively terrestrial species found inside mangrove forests, being more abundant inland, and less abundant closer to the sea. It is also found in the high supra-tidal zone, where sea water submerges the substrate only on a few days each month during the very highest tides. It hides well on the forest floor, moving only at the very last moment when approached. It feeds on small invertebrates. Its behaviour is almost completely unknown.
References
Clayton D.A. (1993) Mudskippers. Oceanography and Marine Bulletin: An Annual Review, 31:507–577.
Diesselhorst G. (1938) Hörversuche an Fischen ohne Weberschen Apparat. Zeitschrift fur Vergleichende Physiologie, 25:748–783.
Evans D.H., Claiborne J.B. and Kormanik G.A. (1999) Osmoregulation, acid-base regulation and nitrogen excretion. In: Horn M.H., Martin K.L.M., Chotkowski M.A. (eds) Intertidal Fishes: Life in Two Worlds. Academic Press, San Diego.
Graham J.B. (1997) Air-Breathing Fishes. Evolution, Diversity and Adaptation. Academic Press, San Diego.
Harris V.A. (1960) On the locomotion of the mud-skipper Periophthalmus koelreuteri (Pallas): (Gobiidae). Proceedings of the Zoological Society of London, 134:107–135.
Ishimatsu A., Yoshida Y., Itoki N., Takeda T., Lee H.J. and Graham J.B. (2007) Mudskippers brood their eggs in air but submerge them for hatching. The Journal of Experimental Biology, 210: 3946–3954.
Ishimatsu A., Hishida Y., Takita T., Kanda T., Oikawa S., Takeda T. and Khoo K.H., (1998) Mudskipper store air in their burrows. Nature, 391: 237–238.
Jaafar Z., Lim K.K.P. and Chou L.M. (2006) Taxonomical and morphological notes on two species of mudskippers, Periophthalmus walailakae and Periophthalmodon schlosseri (Teleostei: Gobiidae) from Singapore. Zoological Science, 23:1043–1047.
Jaafar Z., Perrig M. and Chou L.M. (2009) Periophthalmus variabilis (Teleostei: Gobiidae: Oxudercinae), a valid species of mudskipper, and a re-diagnosis of Periophthalmus novemradiatus. Zoological Science, 26:309–314.
Khaironizam M.Z. and Norma–Rashid Y. (2003) First record of the mudskipper, Periophthalmodon septemradiatus (Hamilton) (Teleostei: Gobiidae) from Peninsular Malaysia. The Raffles Bulletin of Zoology, 51(1):97–100.
Kobayashi T., Dotsu Y. and Miura N. (1972) Egg development and rearing experiments of the larvae of the mud skipper, Periophthalmus cantonensis. Bulletin of the Faculty of Fisheries, Nagasaki University, 35:49–62 [Japanese with English summary].
Lee H.J., Martinez C.A., Hertzberg K.J., Hamilton A.L. and Graham J.B. (2005) Burrow air phase maintenance and respiration by the mudskipper Scartelaos histophorus (Gobiidae: Oxudercinae). The Journal of Experimental Biology, 208: 169–177.
Milward N.E. (1974) Studies on the taxonomy, ecology and physiology of Queensland mudskippers. PhD thesis, University of Queensland, Brisbane.
Monks, Neale (2006). Brackish-Water Fishes: An Aquarist's Guide to Identification, Care & Husbandry, TFH Publications, New Jersey.
Murdy E.O. (1989) A Taxonomic revision and cladistic analysis of the Oxudercine gobies (Gobiidae: Oxudercinae). Records of the Australian Museum,Suppl. No 11:1–93.
Polgar, Gianluca. Themudskipper, World Wide Web electronic publication, www.themudskipper.org (Accessed 06/March/2010).
Polgar, G. and Crosa, G. (2009) Multivariate characterization of the habitats of seven species of Malayan mudskippers (Gobiidae: Oxudercinae). Marine Biology, 156:1475–1486.
Sayer M.D.J. (2005) Adaptations of amphibious fish for surviving life out of water. Fish and Fisheries, 6:186–211.
Stebbins R.C. and Kalk M. (1961) Observations on the natural history of the mud-skipper Periophthalmus sobrinus. Copeia, 1:18–27.
Further information
Please do not hesitate to contact me for any comments or information about mudskippers:
[email protected]
More details on mudskippers are available on my personal website:
http://www.themudskipper.org













